
CerebroMachines Lab
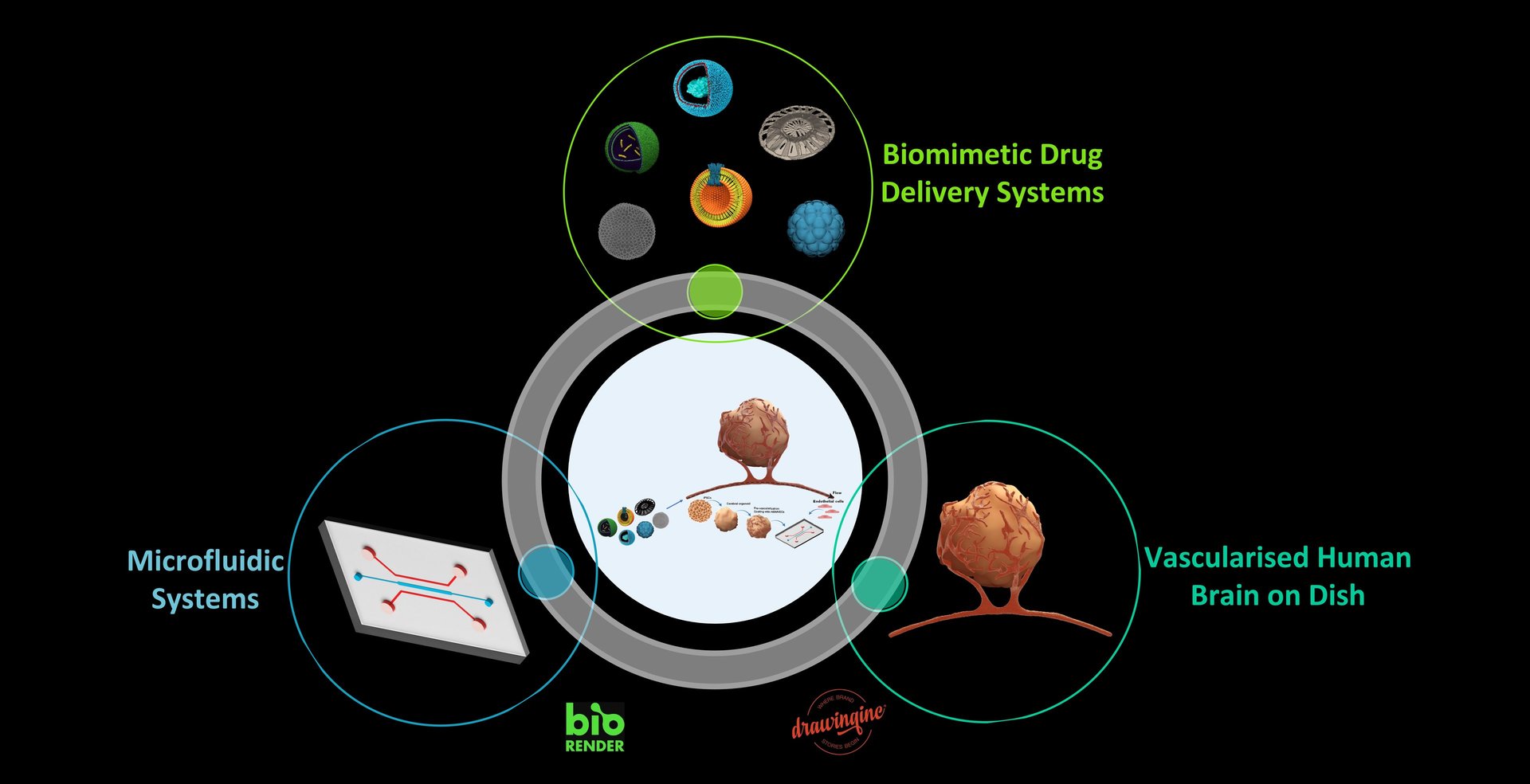
Biomimetic Drug Delivery Systems for Brain / Organ-on-Chip
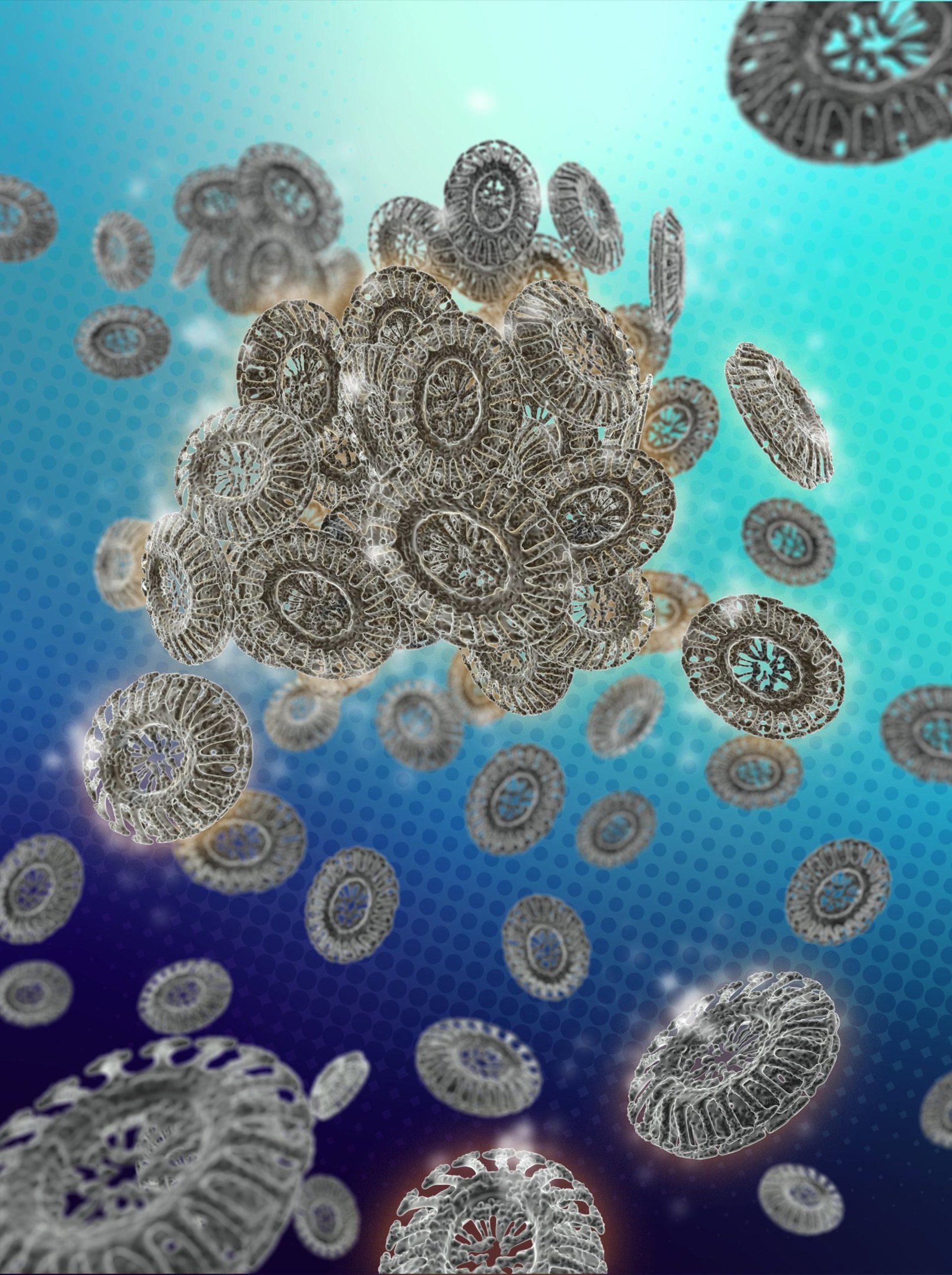
Development of coccolithophore-based and light-driven biohybrid micromotors
Unicellular microalgae intelligently endow new materials with bioinspired geometric intricacy that are challenging to fabricate reproducibly via synthetic approaches. However, microalgae-derived structures require an understanding of the (bio)physicochemical properties that govern their dynamical mechanism if they are to be used in the design/control of cell-mimicking micromotors, which have recently experienced exciting developments. We are deconstructing the coccolithophore cells and use their components (i.e., coccoliths and their cell material) to engineer novel light-driven micromotors.

Synthetic vessels for the control of brain processes and repair
Organoids are complex 3D self-organised cellular entities currently serving as exciting non-animal models. Despite recent progress, organoids are still facing technical challenges in terms of their size and shape control, lack of immune cells, limited power of heterogeneity and cell identity within the organoid composition, limited mature function and lifespan, or even a lack of a concise regulatory framework surrounding the ethical concerns that might arise from their production and deployment. Besides the mentioned challenges, the lack of vasculature within an organoid will provide an impaired biochemical exchange and poor diffusion of nutrients and oxygen within the organoid’s composition that might lead to necrotic cores and directly impact its function and lifespan. Therefore, we are engineering vasculature in 3D disease model organoids using biomaterial-based construct supports and microfluidic cell culture devices to increase the organoid’s lifespan and achieve its designed function, thus unravelling novel mechanistic insights into various disease models.
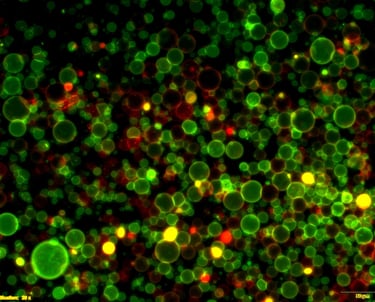
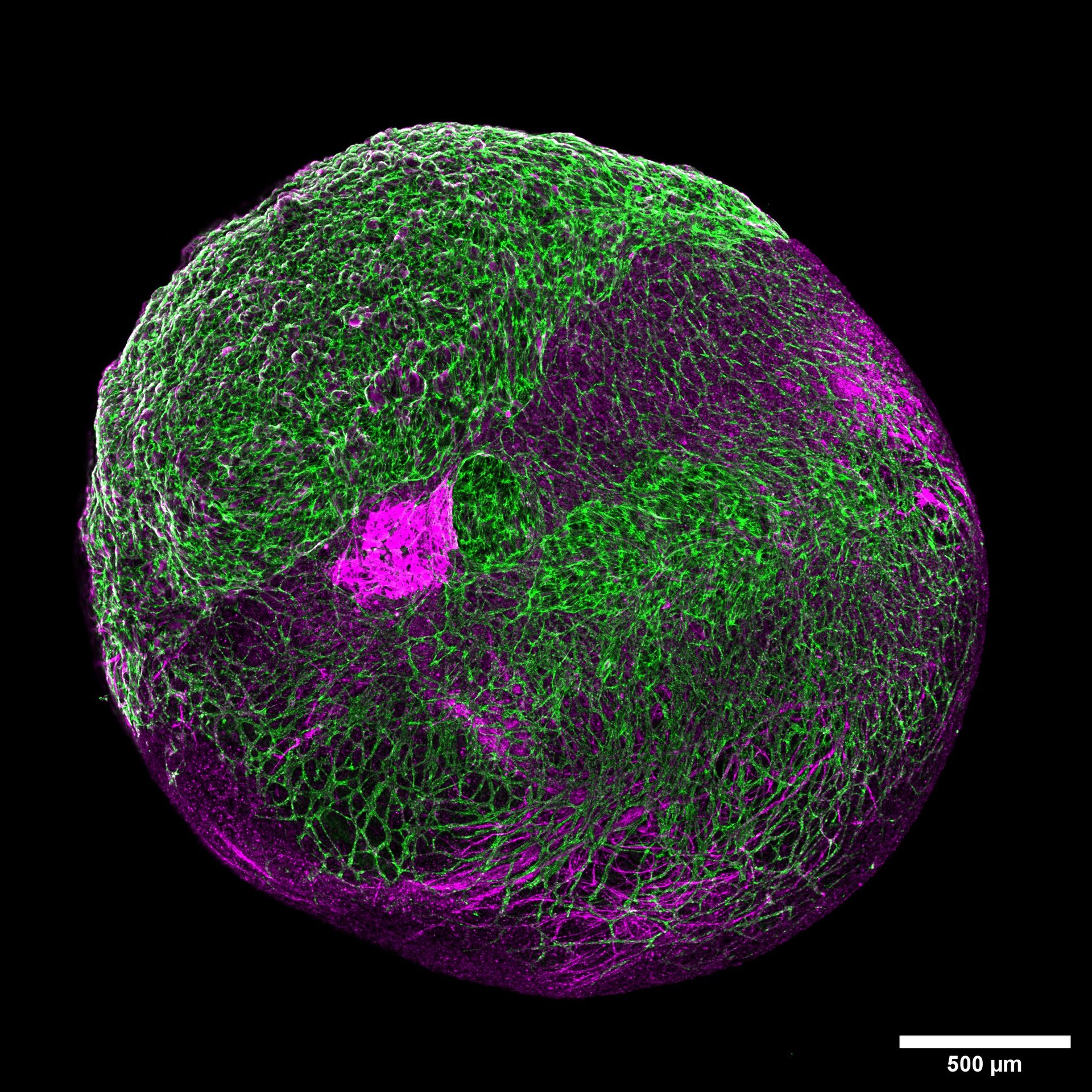
Organoids are complex 3D self-organised cellular entities currently serving as exciting non-animal models. Despite recent progress, organoids are still facing technical challenges in terms of their size and shape control, lack of immune cells, limited power of heterogeneity and cell identity within the organoid composition, limited mature function and lifespan, or even a lack of a concise regulatory framework surrounding the ethical concerns that might arise from their production and deployment. Besides the mentioned challenges, the lack of vasculature within an organoid will provide an impaired biochemical exchange and poor diffusion of nutrients and oxygen within the organoid’s composition that might lead to necrotic cores and directly impact its function and lifespan. Therefore, we are engineering vasculature in 3D disease model organoids using biomaterial-based construct supports and microfluidic cell culture devices to increase the organoid’s lifespan and achieve its designed function, thus unravelling novel mechanistic insights into various disease models.
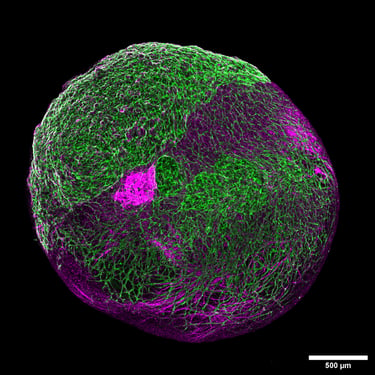
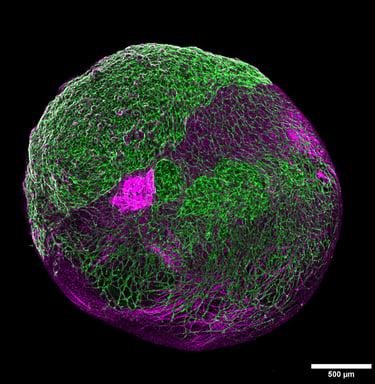
Overcoming the challenges of vasculature in the organoid landscape

Developing an organoid-based stroke model
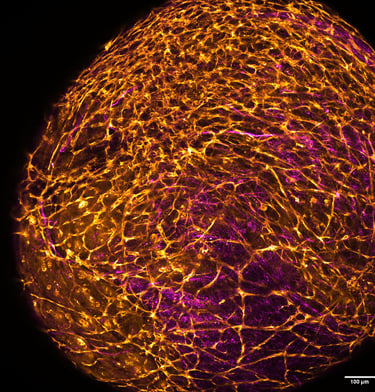
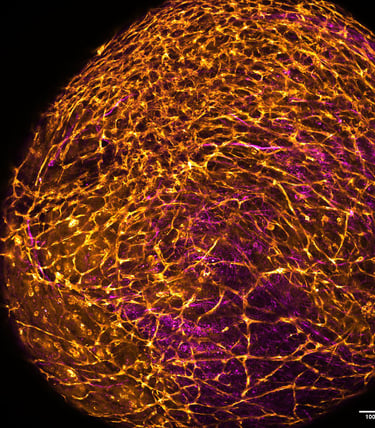
Intracerebral haemorrhage (ICH) is the most devastating subtype of stroke in terms of mortality and morbidity, with limited treatment options available. Preclinical studies are currently performed using animal disease models. However, these fail to recapitulate the full extent of the human condition, and relevant complementary models are required. We are addressing this urgent and unmet need by generating the first multisystem in vitro human model of ICH. This will leverage state-of-the-art methods for co-culturing vascularised cerebral organoids from human induced pluripotent stem cells (hiPSC), encapsulation in biomimetic hydrogels and recent advances in the microfluidic perfusion of complex microtissues. By combining these approaches, we are developing a tractable and scalable platform for disease model development.

Developing a lung-on-chip model
We are using microfluidic chips tailored for lung-on-chip (LoC) applications to develop and validate an Acute Lung Injury (ALI) disease model for drug testing.


The project is part of the Interreg North-West Europe (NWE) STEP4NAMs consortium, which comprises 10 partners collaborating to advance the adoption of new approach methodologies (NAMs) as alternatives to animal testing.
More information about the consortium can be accessed here: link

© 2025. CerebroMachinesLab. All rights reserved.
