
CerebroMachines Lab
Biomimetic Drug Delivery Systems for Brain / Organ-on-Chip
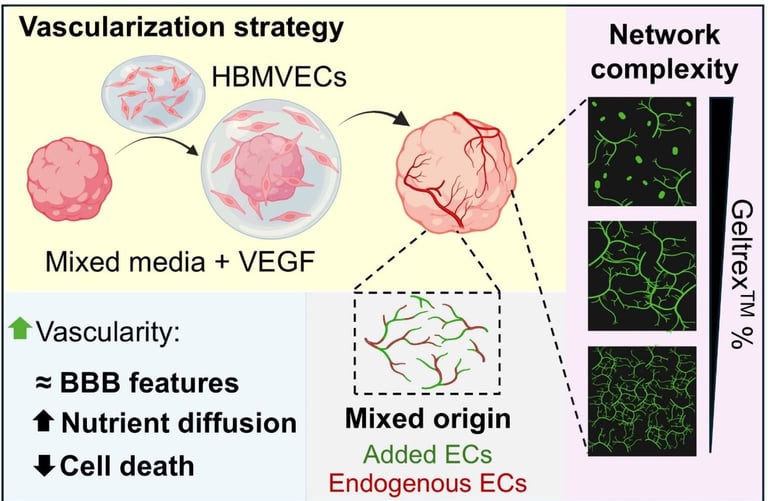
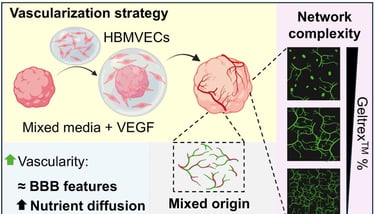
Abstract: Cerebral organoids (COs) are multicellular, self-organized, in vitro, 3D brain-like tissues used for developmental biology, disease modelling and drug screening. However, their lack of vascularity renders them less physiologically accurate. Vascularization of COs remains challenging due to the different requirements between COs and vascular cells, limited vascular network penetration within the organoid, and the absence of luminal perfusion. Here, we devised an encapsulation approach in which human brain microvascular endothelial cells (HBMVECs) were delivered to developing COs from progressively degrading extracellular matrix (ECM)-based hydrogel droplets. By tuning this hydrogel concentration and media composition, we observed enhanced vascular-like network formation that expanded within the organoid tissue. Using pathway inhibitors, we showed that a subset of the endothelial cells (ECs) originated from the CO itself, promoting network integration. Endothelial networks displayed blood-brain barrier (BBB) features, including astrocytic end-footlike interactions, pericyte wrapping, and collagen-laminin basal lamina. Vascularized COs exhibited greater media internalization and up to three-fold lower apoptosis than non-vascularized COs. This comprehensive 3D neurovascular model is a promising platform for cerebrovascular research and drug testing applications.
2025
Fumadó Navarro J., Crilly S., Chan W. K., Browne S., Mason J. O., Giraldo C. V., Pandit A., Lomora M.
Advanced Science, 2025 DOI: 10.1002/advs.202507256
BioRxiv DOI: 10.1101/2025.04.23.650161 )
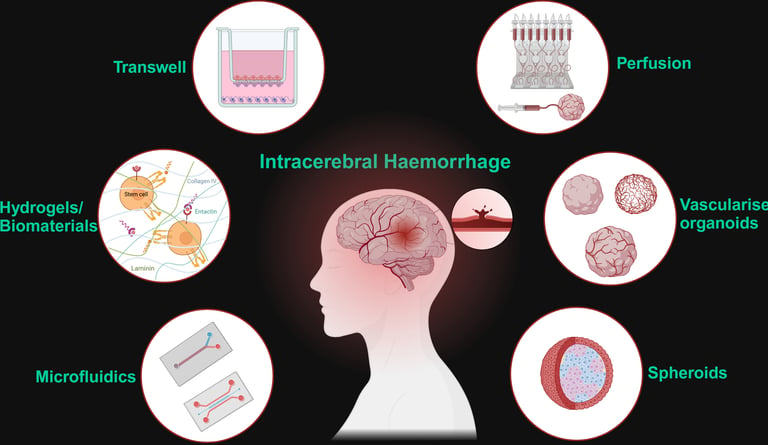
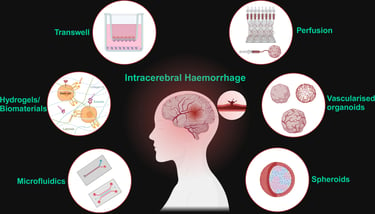
Abstract: Haemorrhagic stroke continues to be a leading cause of death and disability globally, with limited treatment options. Pre-clinical models must adapt to offer translationally relevant and physiologically accurate alternatives to animals. The development of complex co-culture blood-brain barrier models and the incorporation of hydrogels and biomaterials has resulted in microphysical 3D platforms for interrogating drug transport and immune cell transcytosis, however with limited application to haemorrhagic stroke research. Cerebral organoids have revolutionised neuroscience, and novel strategies of vascularising and perfusing them offer a wholly human model system of the cerebrovasculature for the first time. With some adaptation, intracerebral haemorrhage research can benefit from these models for elucidating pathology and recovery mechanisms that can be exploited therapeutically.
2025
Crilly S., Lomora M.
Under Review
KeAi: Bioactive Materials, 2025 (Preprint) DOI: 10.2139/ssrn.5185807
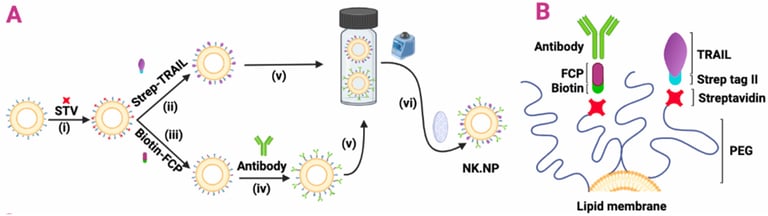
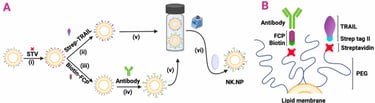
Abstract: Natural killer (NK) cells play a crucial role in recognizing and killing emerging tumor cells. However, tumor cells develop mechanisms to inactivate NK cells or hide from them. Here, we engineered a modular nanoplatform that acts as NK cells (NK cell-mimics), carrying the tumor-recognition and death ligand-mediated tumor-killing properties of an NK cell, yet without being subject to tumor-mediated inactivation. NK cell mimic nanoparticles (NK.NPs) incorporate two key features of activated NK cells: cytotoxic activity via the death ligand, tumor necrosis factor-related
2023
Zeinabad A.H.A, Yeoh W.J., Arif M., Lomora M., Banz Y., Riether C., Krebs P., Szegezdi E.
Biomaterials, 2023. DOI: 10.1016/j.biomaterials.2023.122126
apoptosis-inducing ligand (TRAIL), and an adjustable tumor cell recognition feature based on functionalization with the NK cell Fc-binding receptor (CD16, FCGR3A) peptide, enabling the NK.NPs to bind antibodies targeting tumor antigens. NK.NPs showed potent in vitro cytotoxicity against a broad panel of cancer cell lines. Upon functionalizing the NK.NPs with an anti-CD38 antibody (Daratumumab), NK.NPs effectively targeted and eliminated CD38-positive patient-derived acute myeloid leukemia (AML) blasts ex vivo and were able to target and kill CD38-positive AML cells in vivo, in a disseminated AML xenograft system and reduced AML burden in the bone marrow compared to non-targeted, TRAIL-functionalized liposomes. Taken together, NK.NPs are able to mimicking key antitumorigenic functions of NK cells and warrant their development into nano-immunotherapeutic tools.
as their functionalization with (macro)molecules that are intrinsically mechanoresponsive (e.g., mechanosensitive ion channels and mechanophores). Mechanical forces, including the pathological shear stress and exogenous stimuli (e.g., ultrasound, magnetic fields), used for the activation of mechanoresponsive DDS are also introduced, followed by in vitro and in vivo experimental models used to investigate and validate such novel therapies. Overall, this review aims to propose a fresh perspective through identified challenges and proposed solutions that could be of benefit for the further development of this exciting field.
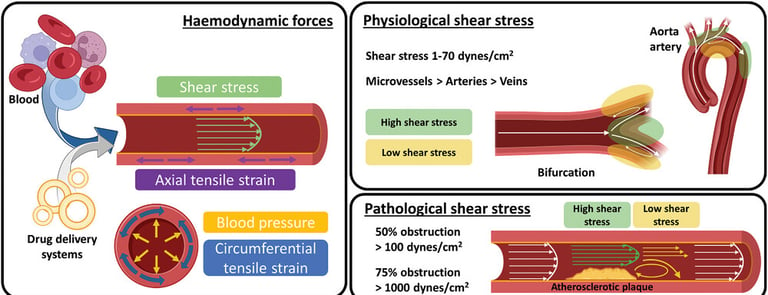
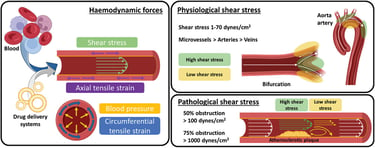
Abstract: Mechanoresponsive drug delivery systems (DDS) have emerged as promising candidates to improve the current effectiveness and lower the side effects typically associated with direct drug administration in the context of vascular diseases. Despite tremendous research efforts to date, designing drug delivery systems able to respond to mechanical stimuli to potentially treat these diseases is still in its infancy. By understanding relevant biological forces emerging in healthy and pathological vascular endothelium, it is believed that better-informed design strategies can be deduced for the fabrication of simple-to-complex macromolecular assemblies capable of sensing mechanical forces. These responsive systems are discussed through insights into essential parameter design (composition, size, shape, and aggregation state) , as well
Abstract: Translating energy into swarming motion for miniature entities remains a challenge. This translation requires simultaneously breaking the symmetry of the system to enable locomotion and a coupling effect between the objects that are part of the population to induce the collective motion. Here, we report on Robocoliths, engineered Emiliania huxleyi (EHUX) coccolith-based miniature hybrid entities capable of swarming behavior. EHUX coccoliths are characterized by an asymmetric morphology that allows breaking symmetry, playing a central role in generating a net force and directed motion. Their activation with the bioinspired material polydopamine not only endows the asymmetric coccoliths with advanced functionalities, such as thermal- and energy-harvesting responsiveness under visible light exposure to display a collective behavior (i.e., swarming), but it also provides a functional surface from which antifouling polymer brushes are grown. In this context, Robocoliths pave the way for the next generation of multifunctional swarming bio-micromachines.
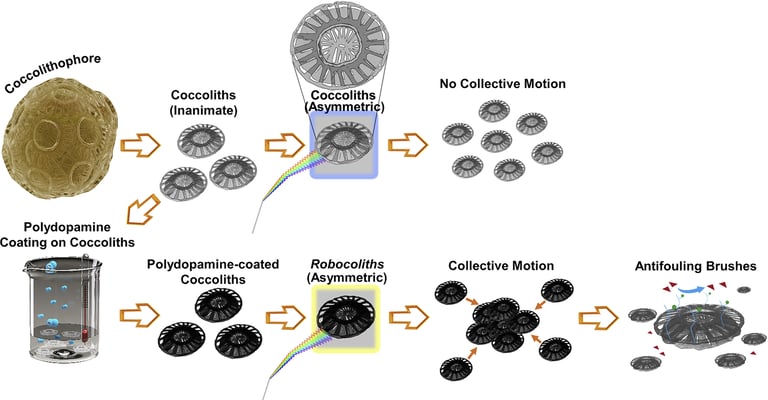
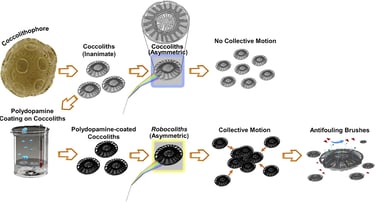
2021
Lomora M., Larrañaga A., Rodriguez-Emmenegger C., Rodriguez B. J., Dinu I.A., Sarasua J.-R., Pandit A.
Cell Reports Physical Science, 2021. DOI: 10.1016/j.xcrp.2021.100373
Huo Z., Lomora M, Kym U., Palivan C., Holland-Cunz S. G., Gros S. J.
Frontiers in Cell and Developmental Biology, 2021. DOI: 10.3389/fcell.2021.605272
Abstract: The water channel aquaporin 1 (AQP1) has been implicated in tumor progression and metastasis. It is hypothesized that AQP1 expression can facilitate the transmembrane water transport leading to changes in cell structure that promote migration. Its impact in neuroblastoma has not been addressed so far. The objectives of this study have been to determine whether AQP1 expression in neuroblastoma is dependent on hypoxia, to demonstrate whether AQP1 is functionally relevant for migration, and to further define AQP1-dependent properties of the migrating cells. This was determined by investigating the reaction of neuroblastoma cell lines, particularly SH-SY5Y, Kelly, SH-EP Tet-21/N and SK-N-BE(2)-M17 to hypoxia, quantitating the AQP1-related water permeability by stopped-flow spectroscopy, and studying the migration-related properties of the cells in a modified transwell assay. We find that AQP1 expression in neuroblastoma cells is up-regulated by hypoxic conditions, and that increased AQP1 expression enabled the cells to form a phenotype which is associated with migratory properties and increased cell agility. This suggests that the hypoxic tumor microenvironment is the trigger for some tumor cells to transition to a migratory phenotype. We demonstrate that migrating tumor cell express elevated AQP1 levels and a hypoxic biochemical phenotype. Our experiments strongly suggest that elevated AQP1 might be a key driver in transitioning stable tumor cells to migrating tumor cells in a hypoxic microenvironment.
Ucar B., Kajtez J., Foidl B. M., Eigel D., Werner C., Long K.R., Emnéus J., Bizeau J., Lomora M., Pandit A., Newland B., Humpel C.
Acta Biomaterialia, 2021. DOI: 10.1016/j.actbio.2020.11.035
Abstract: Protection or repair of the nigrostriatal pathway represents a principal disease-modifying therapeutic strategy for Parkinson's disease (PD). Glial cell line-derived neurotrophic factor (GDNF) holds great therapeutic potential for PD, but its efficacious delivery remains difficult. The aim of this study was to evaluate the potential of different biomaterials (hydrogels, microspheres, cryogels and microcontact printed surfaces) for reconstructing the nigrostriatal pathway in organotypic co-culture of ventral mesencephalon and dorsal striatum. The biomaterials (either alone or loaded with GDNF) were locally applied onto the brain co-slices and fiber growth between the co-slices was evaluated after three weeks in culture based on staining for tyrosine hydroxylase (TH). Collagen hydrogels loaded with GDNF slightly promoted the TH+ nerve fiber growth towards the dorsal striatum, while GDNF loaded microspheres embedded within the hydrogels did not provide an
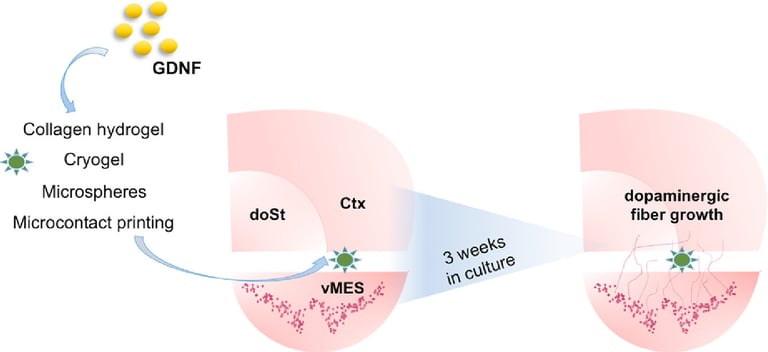
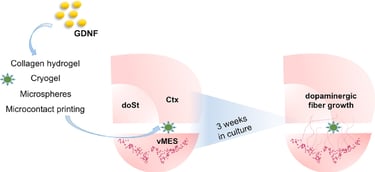
improvement. Cryogels alone or loaded with GDNF also enhanced TH+ fiber growth. Lines of GDNF immobilized onto the membrane inserts via microcontact printing also significantly improved TH+ fiber growth. In conclusion, this study shows that various biomaterials and tissue engineering techniques can be employed to regenerate the nigrostriatal pathway in organotypic brain slices. This comparison of techniques highlights the relative merits of different technologies that researchers can use/develop for neuronal regeneration strategies.
Abstract: Phytoplankton are complex living organisms that have attracted significant interest in the field of biomedicine. One subclass of phytoplankton, the diatoms, produce elegant self-assembled siliceous architectural features with a complex 3D porous structure. Diatoms are characterized by a distinct 3D architecture of silica cell walls called frustules with a highly ordered nano-/micropore structure and pattern. Another phytoplankton subclass of interest is the coccolithophore, which produces unique calcium carbonate plates with distinct architectural features called coccoliths. The unique morphological characteristics of coccoliths resembling the shape of a wagon wheel allows a higher surface area, thus an increased amount of immobilized therapeutic agent on their surface compared to a synthetic calcium carbonate microparticle. This review offers a summary of phytoplankton (microalgae) and their potential for application in drug delivery, diagnostics, drug discovery, as molecular factories, and as scaffolds for various therapeutic applications.
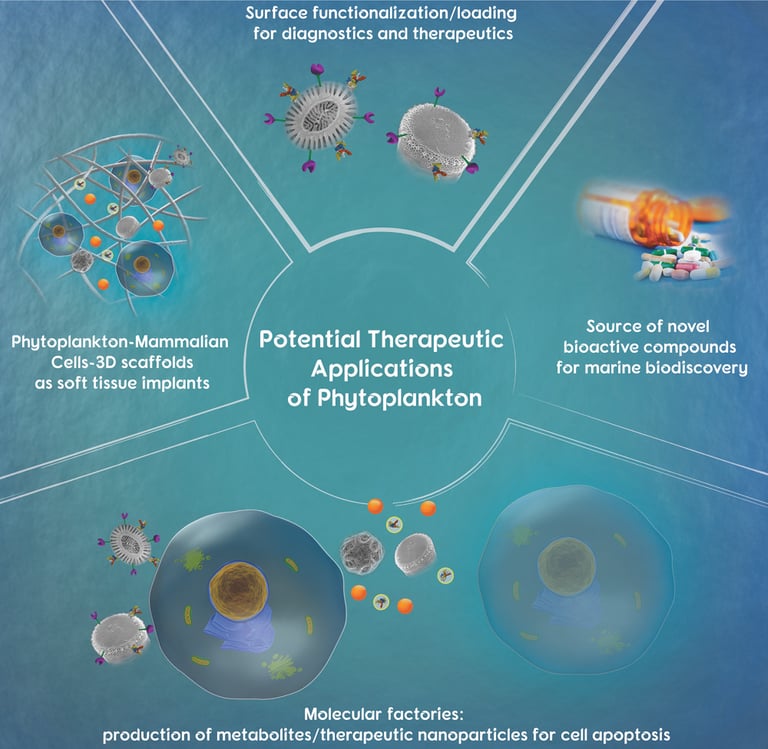
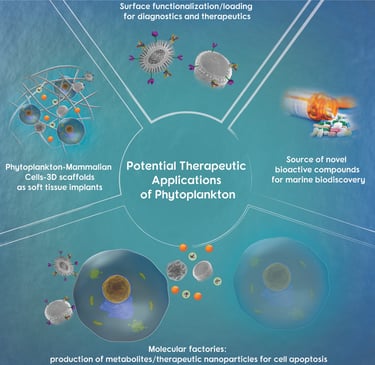
2019
Lomora M., Shumate D., Rahman A. A., Pandit A.
Advanced Therapeutics, 2019. DOI: 10.1002/adtp.201800099
Abstract: In this chapter, a broad overview on recently published literature concerning the design, preparation, and applications of polymer-based smart systems and hybrid structured materials with complex architectures (micelles, vesicles, capsules, gels, and so on) and sizes ranging from nanometers toward several micrometers scale is provided. Aiming the fabrication of smart materials with enhanced properties for applications in biomedicine, the spatial organization and selective confinement of various molecules within the ordered domains of different polymer architectures were also considered. Moreover, the stimuli-responsiveness of these self-organized structures induces drastic changes in their morphology or properties when a specific stimulus is applied, leading to “on demand” release of the cargo or triggering a distinct in situ signal/reaction. Accordingly, results from the most relevant studies are briefly reviewed, more attention being paid to those polymeric systems with potential application in biomedical field, ranging from drug delivery to mimics of natural organelles or other cellular compartments.
2018
Dinu I. A., Dinu M. V., Lomora M., Palivan C. G., Meier W.
in Smart Materials: Integrated Design, Engineering Approaches, and Potential Applications 2018. Apple Academic Press, Eds: Anca Filimon DOI: 10.1201/9781351167963
Larrañaga A., Isa I. L. M., Patil V., Thamboo S., Lomora M., Fernández-Yague M. Y., Sarasua J-R., Palivan C. G., Pandit A.
Acta Biomaterialia, 2018. DOI: 10.1016/j.actbio.2017.12.014
Abstract: Polymeric capsules exhibit significant potential for therapeutic applications as microreactors, where the bio-chemical reactions of interest are efficiently performed in a spatial and time defined manner due to the encapsulation of an active biomolecule (e.g., enzyme) and control over the transfer of reagents and products through the capsular membrane. In this work, catalase loaded polymer capsules functionalized with an external layer of tannic acid (TA) are fabricated via a layer-by-layer approach using calcium carbonate as a sacrificial template. The capsules functionalised with TA exhibit a higher scavenging capacity for hydrogen peroxide and hydroxyl radicals, suggesting that the external layer of TA shows intrinsic antioxidant properties, and represents a valid strategy to increase the overall antioxidant potential of the developed capsules. Additionally, the hydrogen peroxide scavenging capacity of the capsules is enhanced in the presence of the encapsulated catalase. The capsules prevent oxidative stress in an in vitro inflammation model of degenerative disc disease. Moreover, the expression of matrix metalloproteinase-3 (MMP-3), and disintegrin and metalloproteinase with thrombospondin motif-5 (ADAMTS-5), which
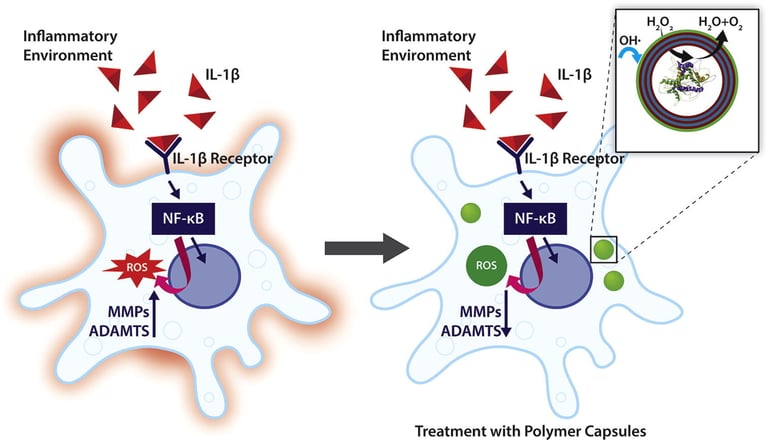
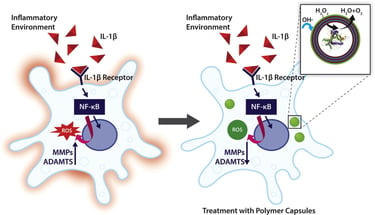
represents the major proteolytic enzymes in intervertebral disc, are attenuated in the presence of the polymer capsules. This platform technology exhibits potential to reduce oxidative stress, a key modulator in the pathology of a broad range of inflammatory diseases.
Abstract: Polymer capsules, fabricated either with the aid of a sacrificial template or via the self-assembly of block copolymers into polymer vesicles (polymersomes), have attracted a great deal of attention for their potential use as micro-/nanoreactors and artificial organelles for therapeutic applications. Compared to other biomedical applications of polymer capsules, such as drug delivery vehicles, where the polymer shell undergoes irreversible disruption/rupture that allows the release of the payload, the polymer shell in polymer micro-/nanoreactors has to maintain mechanical integrity while allowing the selective diffusion of reagents/reaction products. In the present review, strategies that permit precise control of the permeability of the polymer shell while preserving its architecture are documented and critiqued. Together with these strategies, specific examples where these polymer capsules have been employed as micro-/nanoreactors as well as approaches to scale-up and optimize these systems along with future perspectives for therapeutic applications in several degenerative diseases are elucidated.
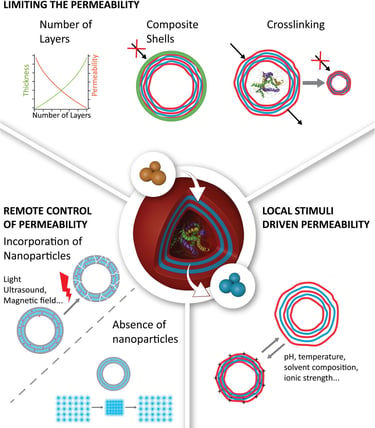
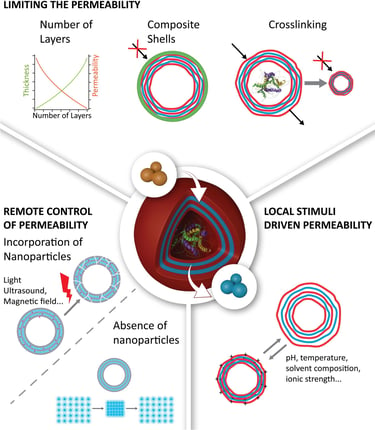
2017
Larrañaga A., Lomora M., Sarasua J.R., Palivan C.G., Pandit A.
Progress in Materials Science, 2017. DOI: 10.1016/j.pmatsci.2017.08.002
2017
Larrañaga A., Lomora M., Sarasua J.R., Palivan C.G., Pandit A.
Progress in Materials Science, 2017. DOI: 10.1016/j.pmatsci.2017.08.002
Lomora M., Gunkel-Grabole G., Mantri S., Palivan C.G.
Chemical Communications, 2017. DOI: 10.1039/C7CC04739H
Abstract: Cells are sophisticated biocatalytic systems driving a complex network of biochemical reactions. A bioinspired strategy to create advanced functional systems is to design confined spaces for complex enzymatic reactions by using a combination of synthetic polymer assemblies and natural cell components. Here, we developed bio-catalytic nanocompartments that contain phosphoglucomutase protected by a biomimetic polymer membrane, which was permeabilized for reactants through insertion of an engineered α-hemolysin pore protein. These bio-catalytic nanocompartments serve for production of glucose-6-phosphate, and thus possess great potential for applications in an incomplete glycolysis, pentose phosphate pathway, or in plant biological reactions.
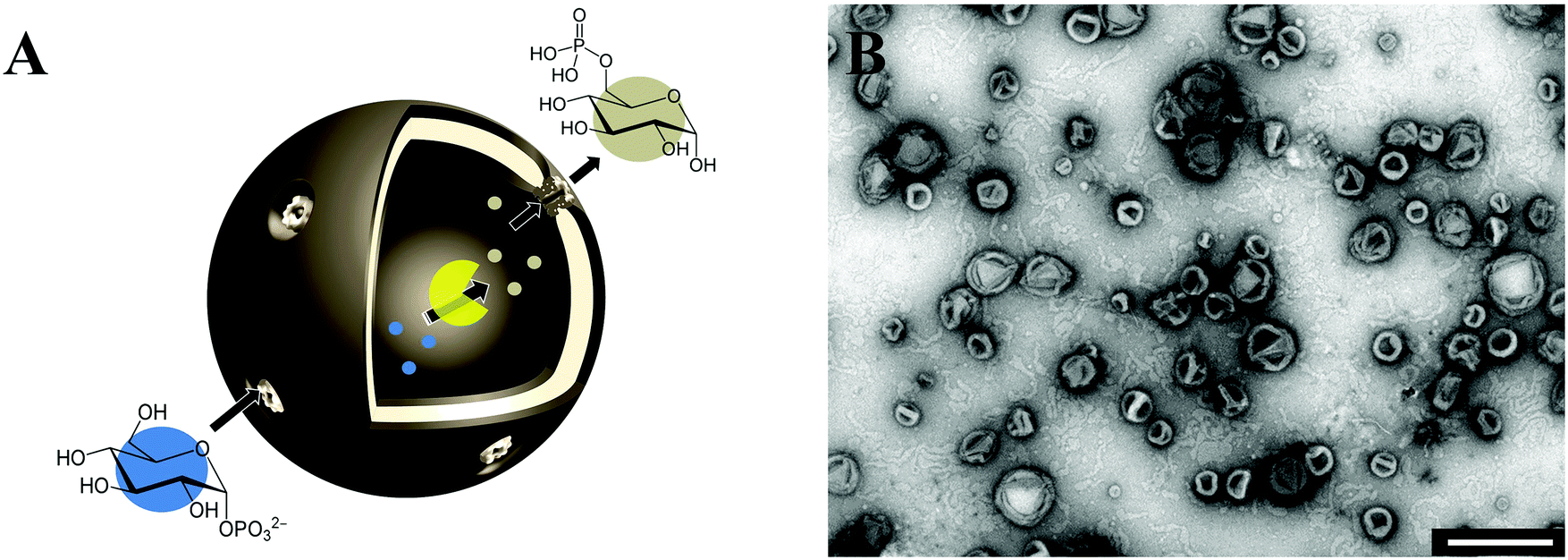

Abstract: Reactions inside confined compartments at the nanoscale represent an essential step in the development of complex multifunctional systems to serve as molecular factories. In this respect, the biomimetic approach of combining biomolecules (proteins, enzymes, mimics) with synthetic membranes is an elegant way to create functional nanoreactors, or even simple artificial organelles, that function inside cells after uptake. Functionality is provided by the specificity of the biomolecule(s), whilst the synthetic compartment provides mechanical stability and robustness. The availability of a large variety of biomolecules and synthetic membranes allows the properties and functionality of these reaction spaces to be tailored and adjusted for building complex self-organized systems as the basis for molecular factories.
2016
Garni M., Einfalt T., Lomora M., Car A., Meier W., Palivan C. G.
Chimia, 2016. DOI: 10.2533/chimia.2016.424
Zhang X., Lomora M., Einfalt T., Meier W., Klein N., Schneider D., Palivan C. G.
Biomaterials, 2016. DOI: 10.1016/j.biomaterials.2016.02.042
Abstract: We introduce active surfaces generated by immobilizing protein-polymer nanoreactors on a solid support for sensitive sugar alcohols detection. First, such selective nanoreactors were engineered in solution by simultaneous encapsulation of specific enzymes in copolymer polymersomes, and insertion of membrane proteins for selective conduct of sugar alcohols. Despite the artificial surroundings, and the thickness of the copolymer membrane, functionality of reconstituted Escherichia coli glycerol facilitator (GlpF) was preserved, and allowed selective diffusion of sugar alcohols to the inner cavity of the polymersome, where encapsulated ribitol dehydrogenase (RDH) enzymes served as biosensing entities. Ribitol, selected as a model sugar alcohol, was detected quantitatively by the RDH-nanoreactors with GlpF-mediated permeability in a concentration range of 1.5–9 mM. To obtain “active surfaces” for detecting sugar alcohols, the nanoreactors optimized in solution were then immobilized on
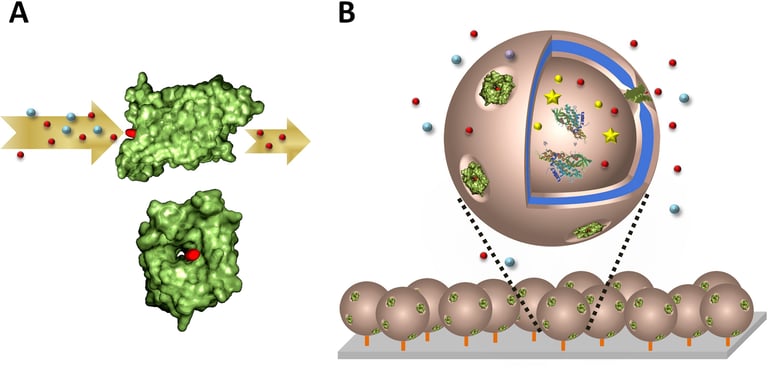
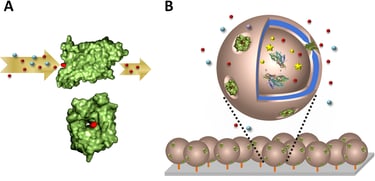
a solid support: aldehyde groups exposed at the compartment external surface reacted via an aldehyde-amino reaction with glass surfaces chemically modified with amino groups. The nanoreactors preserved their architecture and activity after immobilization on the glass surface, and represent active biosensing surfaces for selective detection of sugar alcohols, with high sensitivity.
Zhang X., Lomora M., Einfalt T., Meier W., Klein N., Schneider D., Palivan C. G.
Biomaterials, 2016. DOI: 10.1016/j.biomaterials.2016.02.042
Abstract: Inspired by natural compartments, polymer-based supramolecular assemblies (dendrimers, polymersomes, PICsomes, LBL capsules) have been engineered with a variety of sizes and properties to host biomolecules and thereby yield functional hybrid materials/systems. We present bionanoreactors that are designed by encapsulation/insertion of active compounds (proteins, enzymes, mimics) within the confined space of the assemblies, where these compounds are protected and act in situ. Accessibility to the inner space of the bionanoreactors is a key factor to enable catalytic reactions. For successful biomedical applications, a complex set of requirements regarding bionanoreactors is imposed. Relevant examples of nanoreactors used for the detection and treatment of pathologic conditions are provided, even though they are still in the early stage of research. An overview of this emerging nanoscience-based field and its potential for improved solutions in domains such as medicine are presented.
Postupalenko V., Einfalt T., Lomora M., Dinu I. A., Palivan C. G.
in Organic Nanoreactors: From Molecular to Supramolecular Organic Compounds, 2016. Academic Press, Eds: Samahe Sadjadi. DOI: 10.1016/B978-0-12-801713-5.00011-2
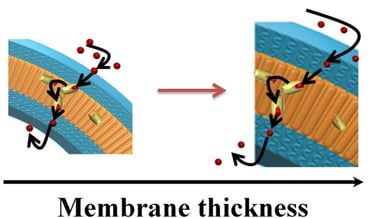
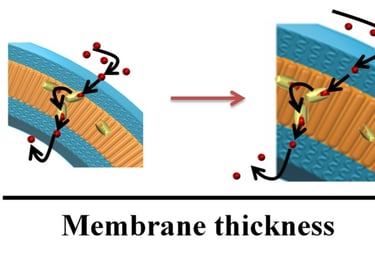
Abstract: Biomimetic polymer nanocompartments (polymersomes) with preserved architecture and ion-selective membrane permeability represent cutting-edge mimics of cellular compartmentalization. Here it is studied whether the membrane thickness affects the functionality of ionophores in respect to the transport of Ca2+ ions in synthetic membranes of polymersomes, which are up to 2.6 times thicker than lipid membranes (5 nm). Selective permeability toward calcium ions is achieved by proper insertion of ionomycin, and demonstrated by using specific fluorescence markers encapsulated in their inner cavities. Preservation of polymersome architecture is shown by a combination of light scattering, transmission electron microscopy, and fluorescence spectroscopy. By using a combination of stopped-flow and fluorescence spectroscopy, it is shown that ionomycin can function and transport calcium ions across polymer membranes with thicknesses in the range 10.7–13.4 nm (7.1–8.9 times larger than the size of the ionophore).
2015
Lomora M., Dinu I.A., Itel F., Rigo S., Spulber M., Palivan C.G.
Macromolecular Rapid Communications, 2015. DOI: 10.1002/marc.201500289
Thicker membranes induce a decrease in transport, but do not block it due to the intrinsic flexibility of these synthetic membranes. The design of ion selective biomimetic nanocompartments represents a new path toward the development of cellular ion nanosensors and nanoreactors, in which calcium sensitive biomacromolecules can be triggered for specific biological functions.
Lomora M., Itel F., Dinu I.A., Palivan C.G.
Physical Chemistry Chemical Physics, 2015. DOI: 10.1039/C4CP05879H
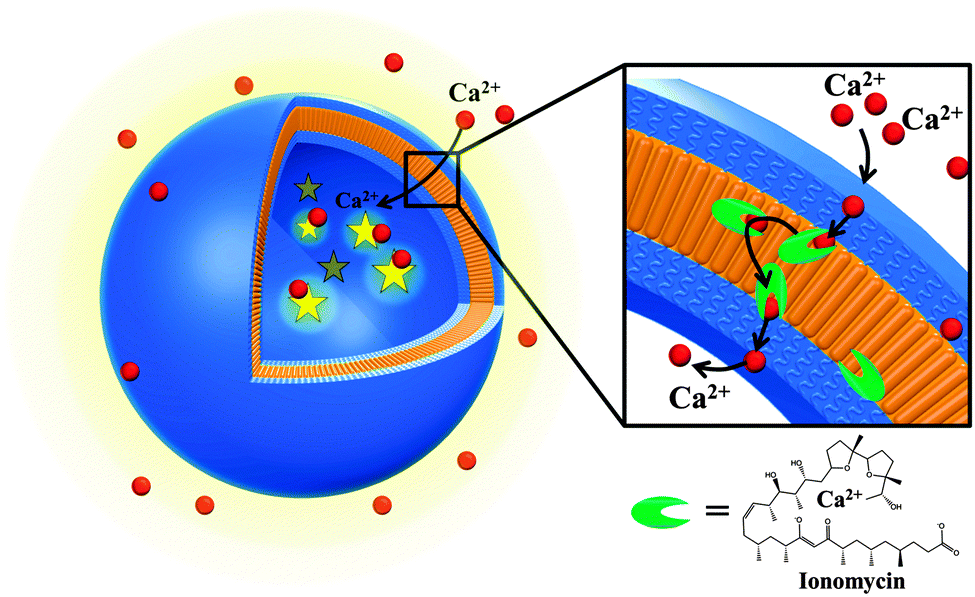
Abstract: In nature there are various specific reactions for which highly selective detection or support is required to preserve their bio-specificity or/and functionality. In this respect, mimics of cell membranes and bio-compartments are essential for developing tailored applications in therapeutic diagnostics. Being inspired by nature, we present here biomimetic nanocompartments with ion-selective membrane permeability engineered by insertion of ionomycin into polymersomes with sizes less than 250 nm. As a marker to assess the proper insertion and functionality of ionomycin inside the synthetic membrane, we used a Ca2+-sensitive dye encapsulated inside the polymersome cavity prior to inserting the biopore. The calcium sensitive dye, ionomycin, and Ca2+ did not influence the architecture and the size of polymersomes. Successful ionomycin functionality inside the synthetic membrane with a thickness of 10.7 nm was established by a combination of fluorescence spectroscopy and stopped-flow spectroscopy. Polymersomes equipped with ion selective membranes are ideal candidates for the development of medical applications, such as cellular ion nanosensors or nanoreactors in which ion exchange is required to support in situ reactions.

Gunkel-Grabole G., Sigg S., Lomora M., Lörcher S., Palivan C. G., Meier W. P.
Biomaterials Science, 2015. DOI: 10.1039/C4BM00230J
Abstract: A promising approach for addressing a range of diseases lies in the delivery of functional biomacromolecules such as nucleic acids or proteins to cells. Polymers, peptides and the different shapes accessible through self-assembly of polymeric and peptidic amphiphiles have been widely explored as carriers and as containers for reactions on the nanoscale. These building blocks are particularly interesting, because several
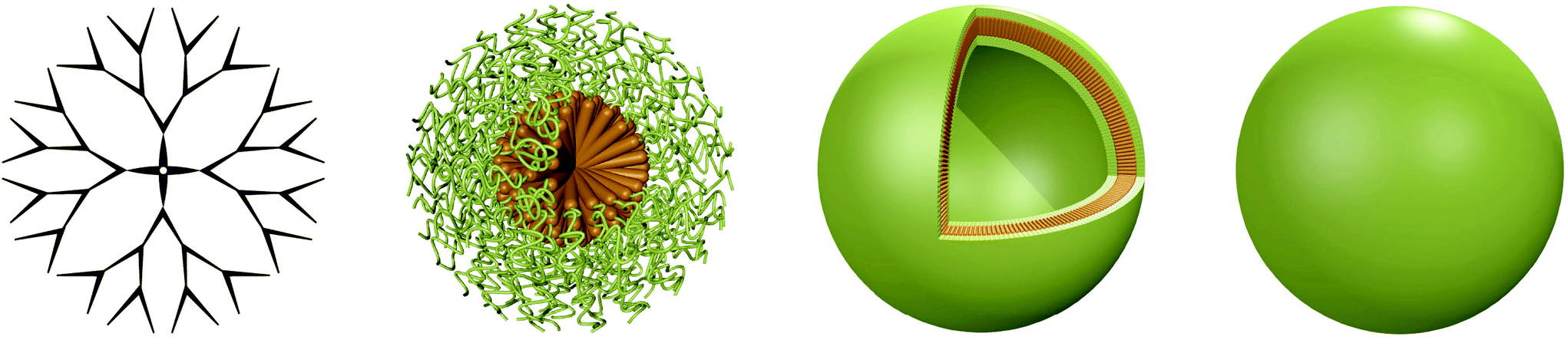

essential parameters such as physical characteristics, conditions for degradation or biocompatibility can be tuned to suit specific requirements. In this review, different three-dimensional architectures ranging from dendrimers and hyperbranched molecules to micelles, vesicles and nanoparticles assembled from synthetic polymers and peptides are discussed. It is focused on their function as a carrier for biologically active macromolecules, highlighting seminal examples from the current literature and pointing out the remaining and upcoming challenges in this important area of research.
Lomora M., Itel F., Dinu I.A., Palivan C.G.
Biomaterials, 2015. DOI: 10.1016/j.biomaterials.2015.02.080
Abstract: Following a biomimetic approach, we present here polymer vesicles (polymersomes) with ion selective permeability, achieved by inserting gramicidin (gA) biopores in their membrane. Encapsulation of pH-, Na+- and K+- sensitive inside the polymersome cavity was used to assess the proper insertion and functionality of gA inside the synthetic membrane. A combination of light scattering, transmission electron microscopy, and fluorescence correlation spectroscopy was used to show that neither the size, nor the morphology of the polymersomes was affected by successful insertion of gA in the polymer membrane. Interestingly, proper insertion and functionality of gA were demonstrated for membranes with thicknesses in the range 9.2 - 12.1 nm, which are significantly greater than membrane lipid counterparts. Both polymersomes with sizes around 100 nm and giant unilamellar vesicles (GUVs) with inserted gA exhibited efficient time response to pH- and ions and therefore are ideal candidates for designing nanoreactors or biosensors for a variety of applications in which changes in the environment, such as variations of ionic concentration or pH, are required.
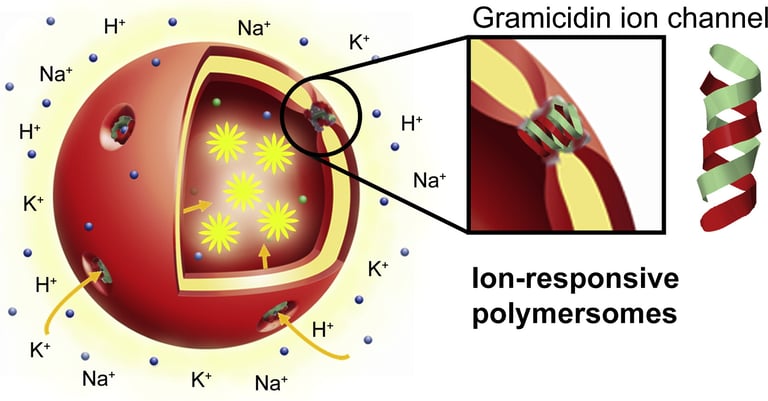
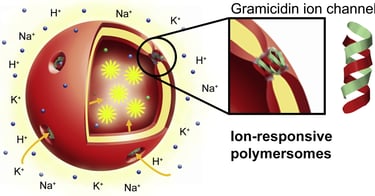
Lomora M., Itel F., Dinu I.A., Palivan C.G.
Biomaterials, 2015. DOI: 10.1016/j.biomaterials.2015.02.080

© 2025. CerebroMachinesLab. All rights reserved.
